
Before balancing the oxygen along with hydrogen, it is vital to check the atoms as there will be a requirement to utilise the coefficients for balancing the other atoms as well in the equation.
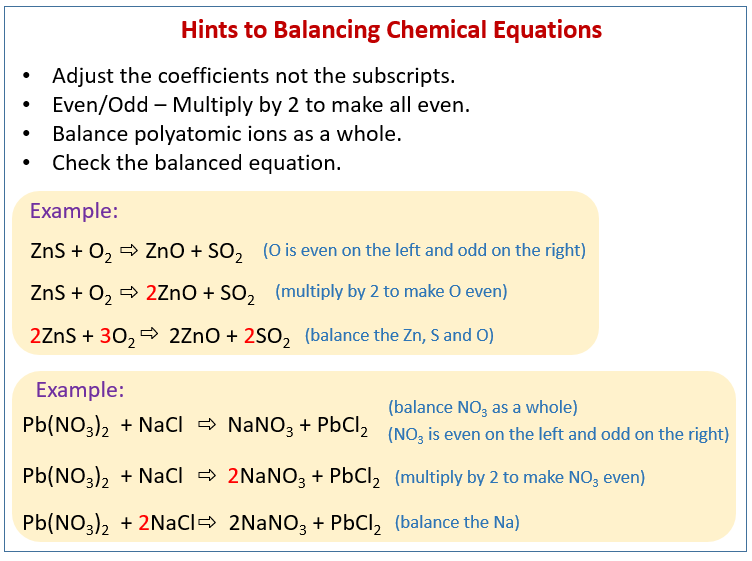
Thus it is best to save them for the last and balance at last. In the molecules, oxygen and hydrogen both are common and it is probable that they may appear in both the sides of the equation.

According to the law of conservation of mass, in a chemical reaction, no atoms can be made or demolished therefore the number of atoms which are existent in the reactants should be balanced with the number of atoms which are existing in the products.
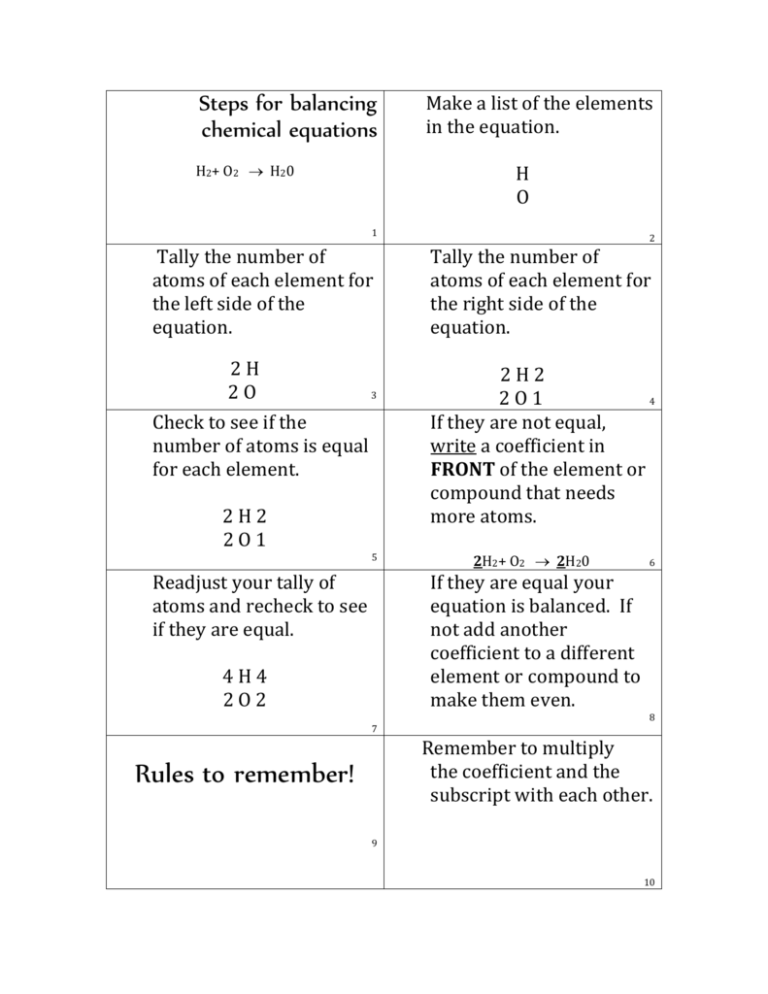
These two are linked through an arrow prominent from left to right for symbolising the reaction. On the left side, the reactant chemicals are specified along with the product chemicals which are mentioned on the right-hand side. The symbols are represented by an alphabet or combination of letters signifying that element. A written symbolic depiction of a chemical reaction is referred to as a Chemical Equation.


 0 kommentar(er)
0 kommentar(er)
